Characterization of microvesicles released from human red blood cells
http://repository.vnu.edu.vn/handle/VNU_123/32644
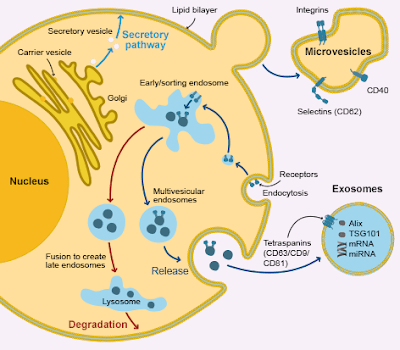
Extracellular vesicles (EVs) are spherical fragments of cell membrane released from various cell types under physiological as well as pathological conditions.
Based on their size and origin, EVs are classified as exosome, microvesicles (MVs) and apoptotic bodies.
Recently, the release of MVs from human red blood cells (RBCs) under different conditions has been reported. MVs are released by outward budding and fission of the plasma membrane.
However, the outward budding process itself, the release of MVs and the physical properties of these MVs have not been well investigated.
The aim of this study is to investigate the formation process, isolation and characterization of MVs released from RBCs under conditions of stimulating Ca2+ uptake and activation of protein kinase C.
Methods: Experiments were performed based on single cell fluorescence imaging, fluorescence activated cell sorter/flow cytometer (FACS), scanning electron microscopy (SEM), atomic force microscopy (AFM) and dynamic light scattering (DLS).
The released MVs were collected by differential centrifugation and characterized in both their size and zeta potential.
Results: Treatment of RBCs with 4-bromo-A23187 (positive control), lysophosphatidic acid (LPA), or phorbol-12 myristate-13 acetate (PMA) in the presence of 2 mM extracellular Ca2+ led to an alteration of cell volume and cell morphology.
In stimulated RBCs, exposure of phosphatidylserine (PS) and formation of MVs were observed by using annexin V-FITC.
The shedding of MVs was also observed in the case of PMA treatment in the absence of Ca2+, especially under the transmitted bright field illumination.
By using SEM, AFM and DLS the morphology and size of stimulated RBCs, MVs were characterized. The sizes of the two populations of MVs were 205.8 ± 51.4 nm and 125.6 ± 31.4 nm, respectively. Adhesion of stimulated RBCs and MVs was observed.
The zeta potential of MVs was determined in the range from - 40 mV to - 10 mV depended on the solutions and buffers used.
Conclusion: An increase of intracellular Ca2+ or an activation of protein kinase C leads to the formation and release of MVs in human RBCs.
Extracellular vesicles (EVs) are spherical fragments of cell membrane released from various cell types under physiological as well as pathological conditions.
Based on their size and origin, EVs are classified as exosome, microvesicles (MVs) and apoptotic bodies.
Recently, the release of MVs from human red blood cells (RBCs) under different conditions has been reported. MVs are released by outward budding and fission of the plasma membrane.
However, the outward budding process itself, the release of MVs and the physical properties of these MVs have not been well investigated.
The aim of this study is to investigate the formation process, isolation and characterization of MVs released from RBCs under conditions of stimulating Ca2+ uptake and activation of protein kinase C.
Methods: Experiments were performed based on single cell fluorescence imaging, fluorescence activated cell sorter/flow cytometer (FACS), scanning electron microscopy (SEM), atomic force microscopy (AFM) and dynamic light scattering (DLS).
The released MVs were collected by differential centrifugation and characterized in both their size and zeta potential.
Results: Treatment of RBCs with 4-bromo-A23187 (positive control), lysophosphatidic acid (LPA), or phorbol-12 myristate-13 acetate (PMA) in the presence of 2 mM extracellular Ca2+ led to an alteration of cell volume and cell morphology.
In stimulated RBCs, exposure of phosphatidylserine (PS) and formation of MVs were observed by using annexin V-FITC.
The shedding of MVs was also observed in the case of PMA treatment in the absence of Ca2+, especially under the transmitted bright field illumination.
By using SEM, AFM and DLS the morphology and size of stimulated RBCs, MVs were characterized. The sizes of the two populations of MVs were 205.8 ± 51.4 nm and 125.6 ± 31.4 nm, respectively. Adhesion of stimulated RBCs and MVs was observed.
The zeta potential of MVs was determined in the range from - 40 mV to - 10 mV depended on the solutions and buffers used.
Conclusion: An increase of intracellular Ca2+ or an activation of protein kinase C leads to the formation and release of MVs in human RBCs.
| Title: | Characterization of microvesicles released from human red blood cells |
| Authors: | Nguyen, D.B. Thuy Ly, T.B. Wesseling, M.C., (...) Perrie, Y. Bernhardt, I. |
| Keywords: | Cell adhesion Fluorescence imaging Microvesicles Phosphatidylserine Red blood cells |
| Issue Date: | 2016 |
| Publisher: | S. Karger AG |
| Citation: | Scopus |
| Abstract: | Extracellular vesicles (EVs) are spherical fragments of cell membrane released from various cell types under physiological as well as pathological conditions. Based on their size and origin, EVs are classified as exosome, microvesicles (MVs) and apoptotic bodies. Recently, the release of MVs from human red blood cells (RBCs) under different conditions has been reported. MVs are released by outward budding and fission of the plasma membrane. However, the outward budding process itself, the release of MVs and the physical properties of these MVs have not been well investigated. The aim of this study is to investigate the formation process, isolation and characterization of MVs released from RBCs under conditions of stimulating Ca2+ uptake and activation of protein kinase C. Methods: Experiments were performed based on single cell fluorescence imaging, fluorescence activated cell sorter/flow cytometer (FACS), scanning electron microscopy (SEM), atomic force microscopy (AFM) and dynamic light scattering (DLS). The released MVs were collected by differential centrifugation and characterized in both their size and zeta potential. Results: Treatment of RBCs with 4-bromo-A23187 (positive control), lysophosphatidic acid (LPA), or phorbol-12 myristate-13 acetate (PMA) in the presence of 2 mM extracellular Ca2+ led to an alteration of cell volume and cell morphology. In stimulated RBCs, exposure of phosphatidylserine (PS) and formation of MVs were observed by using annexin V-FITC. The shedding of MVs was also observed in the case of PMA treatment in the absence of Ca2+, especially under the transmitted bright field illumination. By using SEM, AFM and DLS the morphology and size of stimulated RBCs, MVs were characterized. The sizes of the two populations of MVs were 205.8 ± 51.4 nm and 125.6 ± 31.4 nm, respectively. Adhesion of stimulated RBCs and MVs was observed. The zeta potential of MVs was determined in the range from - 40 mV to - 10 mV depended on the solutions and buffers used. Conclusion: An increase of intracellular Ca2+ or an activation of protein kinase C leads to the formation and release of MVs in human RBCs. |
| Description: | Cellular Physiology and Biochemistry Volume 38, Issue 3, 1 March 2016, Pages 1085-1099 |
| URI: | https://www.karger.com/Article/FullText/443059 http://repository.vnu.edu.vn/handle/VNU_123/32644 |
| ISSN: | 10158987 |
| Appears in Collections: | Bài báo của ĐHQGHN trong Scopus |


Nhận xét
Đăng nhận xét